ʻO Tofacitinib citrate kahi lāʻau lapaʻau (inoa kālepa Xeljanz) i hoʻokumu mua ʻia e Pfizer no kahi papa o ka waha o Janus kinase (JAK) inhibitors. Hiki iā ia ke hoʻokaʻawale i ka JAK kinase, e ālai i nā ala JAK / STAT, a ma laila e pale ai i ka transduction hōʻailona cell a me ka hōʻike gen pili a me ka hoʻōla ʻana, i hoʻohana ʻia e mālama i ka rheumatoid arthritis, psoriatic arthritis, ulcerative colitis a me nā maʻi ʻē aʻe.
Aia ka lāʻau lapaʻau i ʻekolu mau ʻano dosage: nā papa, nā papa hoʻokuʻu hoʻomau a me nā hoʻonā waha. Ua ʻae mua ʻia kāna mau papa e ka FDA i ka makahiki 2012, a ua ʻae ʻia ka palapala hoʻokuʻu mau ʻia e ka FDA i Pepeluali 2016. ʻO ia ka mea mua e mālama i nā hui rheumatoid. ʻO Yan kahi mea pale JAK i lawe waha ʻia i hoʻokahi lā i ka lā. I Kekemapa 2019, ua ʻae hou ʻia kahi hōʻailona hou no nā lāʻau hoʻokuʻu hoʻomau ʻia no ka maʻi ulcerative colitis (UC). Eia kekahi, ua hoʻopau ʻia nā hoʻokolohua lapaʻau o kēia manawa 3 no ka psoriasis plaque, a ke holomua nei nā hoʻokolohua hoʻāʻo ʻeono ma ka pae 3, e pili ana i ka maʻi psoriatic ikaika, ka maʻi o ka idiopathic juvenile, a pēlā aku. ʻO nā mea maikaʻi o nā papa hoʻokuʻu hoʻomau lōʻihi a pono e lawe ʻia i hoʻokahi manawa i ka lā e kūpono i ka mālama ʻana a me ka mālama ʻana i nā maʻi o nā maʻi.
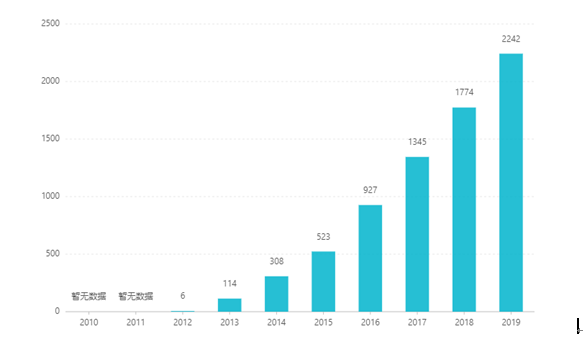
Mai kona papa inoa ʻana, ua hoʻonui ʻia kāna kūʻai ʻana i kēlā me kēia makahiki, a hiki i ka US $ 2.242 biliona ma 2019. Ma Kina, ua ʻae ʻia ka palapala dosage papa no ke kūʻai aku ʻana ma Malaki 2017, a ua komo i loko o ka waihona ʻinikua olakino B ma o ke kūkākūkā ʻana ma 2019. ʻO ka lanakila hou loa. ʻO ke kumu kūʻai he RMB 26.79. Eia nō naʻe, ma muli o nā pale ʻenehana kiʻekiʻe o ka hoʻomākaukau hoʻokuʻu hoʻomau, ʻaʻole i kūʻai ʻia kēia ʻano dosage ma Kina.
He kuleana koʻikoʻi ka JAK kinase i ka ʻāʻī, a ua hōʻike ʻia kāna mau mea paʻa e mālama i kekahi mau maʻi ʻeha a me nā maʻi autoimmune. A hiki i kēia manawa, ua ʻae ʻia ka 7 JAK inhibitors ma ka honua holoʻokoʻa, me Leo Pharma's Delgocitinib, Celgene's Fedratinib, AbbVie's upatinib, Astellas's Pefitinib, Eli Lilly's Baritinib A me Novartis's Rocotinib. Eia nō naʻe, ʻo tofacitinib, baritinib a me rocotinib wale nō i ʻae ʻia ma Kina ma waena o nā lāʻau lapaʻau i ʻōlelo ʻia ma luna. Manaʻo mākou i ka Qilu's "Tofatib Citrate Sustained Release Tablets" i ʻae ʻia i ka wā hiki loa a e pōmaikaʻi ai i nā maʻi hou aʻe.
Ma Kina, ua ʻae ʻia ka noiʻi mua tofacitib citrate e ka NMPA i Malaki 2017 no ka mālama ʻana i nā poʻe maʻi RA makua me ka lawa ʻole a i ʻole intolerance i ka methotrexate, ma lalo o ka inoa kālepa ʻo Shangjie. Wahi a ka ʻikepili mai Meinenet, ʻo ke kūʻai aku ʻana o nā papa citrate tofacitib i nā keʻena olakino lehulehu o Kina i ka makahiki 2018 he 8.34 miliona yuan, ʻoi aku ka haʻahaʻa ma mua o kāna kūʻai honua. ʻO kahi hapa nui o ke kumu ke kumu kūʻai. Ua hōʻike ʻia ʻo ke kumukūʻai kūʻai mua o Shangjie he 2085 yuan (5mg * 28 papa), a ʻo ke kumukūʻai o kēlā me kēia mahina he 4170 yuan, ʻaʻole ia he ukana liʻiliʻi no nā ʻohana maʻamau.
Eia naʻe, pono e hoʻolauleʻa i ka tofacitib i hoʻokomo ʻia i ka 2019 "National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List" e ka National Medical Insurance Administration ma hope o ke kūkākūkā ʻana ma Nowemapa 2019. Ua hōʻike ʻia e hoʻemi ʻia ka uku o kēlā me kēia mahina. i lalo o 2,000 yuan ma hope o ke kūkākūkā ʻana o ke kumukūʻai, e hoʻomaikaʻi nui i ka loaʻa ʻana o ka lāʻau.
Ma August 2018, ka Patent Reexamination Board o ka Moku'āina Intellectual Property Office i ka loiloi hoʻoholo No. 36902 noi no ka invalidation, a hai aku i ka pono ole ka patent kumu o Pfizertofatib, ka patent hui, ma ke kumu o ka lawa ole ka hoike ana o ka hoakaka. Eia naʻe, e pau ka patent o Pfizertofatiib crystal form (ZL02823587.8, CN1325498C, lā noi 2002.11.25) i 2022.
Hōʻike ka ʻikepili Insight, ma waho aʻe o ka noiʻi mua, ʻelima mau lāʻau lapaʻau o Chia Tai Tianqing, Qilu, Kelun, Yangtze River, a me Nanjing Chia Tai Tianqing i ʻae ʻia no ke kūʻai aku ʻana i nā ʻano papa tofacitinib home. Eia nō naʻe, no ke ʻano papa hoʻokuʻu hoʻomau, ʻo ka noiʻi mua wale nō ʻo Pfizer i hoʻouna i kahi noi kūʻai ma Mei 26. ʻO Qilu ka hui kūloko mua e hoʻouna i kahi noi kūʻai no kēia hoʻolālā. Eia kekahi, aia ʻo CSPC Ouyi i ka pae hoʻokolokolo BE.
ʻO Changzhou Pharmaceutical Factory (CPF) kahi mea hana lāʻau lapaʻau koʻikoʻi o nā API, i hoʻopau ʻia i loko o Kina, aia ma Changzhou, ka mokuʻo Jiangsu. Ua hoʻokumu ʻia ʻo CPF ma 1949. Ua hoʻolaʻa mākou i Tofacitinib Citrate mai 2013, a ua waiho mua iā DMF. Ua kākau inoa mākou ma nā ʻāina he nui, a hiki iā mākou ke kākoʻo iā ʻoe me ke kākoʻo palapala maikaʻi loa no Tofacitinib Citrate.
Ka manawa hoʻouna: Iulai-23-2021